SPECT-CT Imaging Unit
Single photon emission computed tomography (SPECT) imaging is a versatile imaging modality complementary to others like PET or MRI. SPECT/CT is versatile non-invasive molecular and morphological functional imaging technique with great translational potential in biomedical research and drug development. It is characterised by the in vivo detection of tracers, labelled with a γ-emitting isotope (25-365 keV), and administrated to live animals at levels well under their pharmacologically active concentrations (high sensitivity). Tracers may bind to a specific protein in specific tissue in the body, or to drugs or to delivery systems. Tracers are followed in real time throughout the whole body and information on target affinity, kinetics, distribution and disposition can be obtained. SPECT/CT is able to quantify changes in tumour size, cardiovascular function, drug distribution and delivery, neurotransmission capacity, disposition routes, pharmacokinetics/ dynamics, etc. The changes can be followed longitudinally in a single animal, which eliminates the need for studying large animal groups, free of post-mortem/tissue dissection artefacts.
The average researcher usually needs a bit of help in realising what the technology can offer and how can this be achieved.
Contact person: J. Arturo García-Horsman, Tel +358-2941-59461 (GSM +358-50-4480761), arturo.garcia@helsinki.fi Location: Biocentre3, Viikinkaari 1, Viikki Campus, University of Helsinki
Services:
- In vivo preclinical multi-pinhole SPECT/CT Imaging.
- High detection sensitivity and spatial resolution (≤0.5mm, depending on collimators)
-
Simultaneously imaging of two or three differnt labels (with different energy peaks)
-
Computed tomography (CT) for anatomical information
- Auto-fusion of SPECT and CT in 3D.
- Preclinical imaging on disease models
- Ex vivo distribution validation
- Tracer preparation (in collaboration with HiLIFE infrastructure Radiopharmaceutical chemistry)
- Consultation in SPECT/CT imaging and related radiolabelling questions
Instruments:
- nanoSPECT/CT camera system with four detectors, and multi-pinhole collimators for the most efficient detection. This makes the system with the highest resolution in the market.
- Workstation with state-of-the-art Tera-Tomo™ 3D SPECT reconstruction software which includes CT correction
- Class B radiation laboratory for in-house radiotracer preparation
Applications:
SPECT/CT is suitable for applications in drug research, neuroscience, oncology, and others as:
- Bio-distribution studies of a single molecule, a delivery system, or a vehicle (separate or in parallel)
- Drug delivery
- Drug pharmacodynamics/pharmacokinetics
- Target validation
- Neurotransmitter-specific neuronal integrity
- Tumour integrity
Commercial Radiotracers
|
Radiotracer |
Target |
Application |
|
123I-ADAM ([123I]2-((2- ((dimethylamino)methyl)phenyl) thio)-5-iodophenylamine). |
Serotonin transporters |
Serotoninergic density |
|
99mTc Sodium pertechnetate |
Multiple |
-Brain scintigraphy -Scintigraphy of thyroid and salivary glands -Scintigraphy of stomach -Arthroscintigraphy |
|
123I-β-CIT ([123I]2ß-carbomethoxy-3ß-(4-iodophenyl) tropane) |
Dopamine transporter |
-Dopaminergic neuron density (also binds to SERT with distinguishable kinetics) |
|
123I-Epidepride |
Dopamine-D2 |
Imaging of brain postsynaptic densities |
|
125I- A-85380 |
Alphabeta neuronal nicotinic acetylcholine receptor |
Neurodegeneration |
|
125I-AB-MECA (4-Amino-3-[125I]iodobenzyl-5`-N-)- |
A3 adenosine receptor |
breast cancer, experimental |
|
DaTSCAN ([123I]-Ioflupane) |
Dopamine Transporter |
Dopaminergic system presynaptic tracer |
|
Xenon-133 (133Xe) |
- |
Cerebral blood flow |
|
123I- IMP (amine N-isopropyl-[123I] iodoamphetamine) |
- |
Cerebral blood flow, acute stroke. |
|
99mTc-HMPAO ([99mTc], hexamnethyl-propyleneamine-oxime) |
- |
Cerebral blood flow |
|
99mTc-ECD ([99mTc] ethyl cysteinate dimmer) |
- |
Cerebral blood flow |
|
99mTc diphosphonate |
Osteocyte/Calcium |
Bone anatomy/physiology |
|
201Tl (thallium-201) |
i.e., Na-K-ATPase, non-energy-dependent cotransporter, etc. |
Myocardial perfusion imaging |
|
99mTc-sestamibi |
P-glycoprotein |
Parathyroid gland |
|
99mTc-tetrofosmin |
P-glycoprotein |
Myocardial perfusion imaging |
|
111In-labeled Octreotide |
Somatostatin receptor |
Thymic tumours |
|
111In-oxine |
Leukocyte/stem cell labelling |
BBB integrity, stem cell migration |
|
[125I]-CLINDE 6-chloro-2-(4′iodophenyl)-3-(N,N-diethyl)-imidazo[1,2-a]pyridine-3-acetamide |
Translocator protein (peripheral-type benzodiazepine receptor) |
Neuroinflammation |
User fees:
The research infrastructure is supported by University of Helsinki (HiLIFE, Faculty of Pharmacy) and Biocenter Finland. The academic prices (excl. VAT) are listed below. Please, ask a quotation for your project. Industrial customers, please contact García-Horsman for prices.
|
Service |
€/h |
€/day |
|
SPECT/CT scan |
110 |
550 |
|
Other expenses |
Commercially available tracers* charged at provider’s price (e.g. [111]-Indium for cell labelling around 380€/batch) plus labour (60 €/h) plus QC radiochemistry laboratory fee (40€/hour). Rodents are available internally or from external provider and prices depends on species, strain, and age. (Admission of animals from the customer should ensure our standards on pathogen status). Animal housing charges might also be applied (0.5-1.50 €/day), as well as veterinary service wrking time, depending on the project specifics. |
|
*Especial tracer synthesis is oursurced to the Radiopharmaceutical Chemistry laboratory.
Real-Time Imaging Unit (RTI) – SPECT/CT in detail
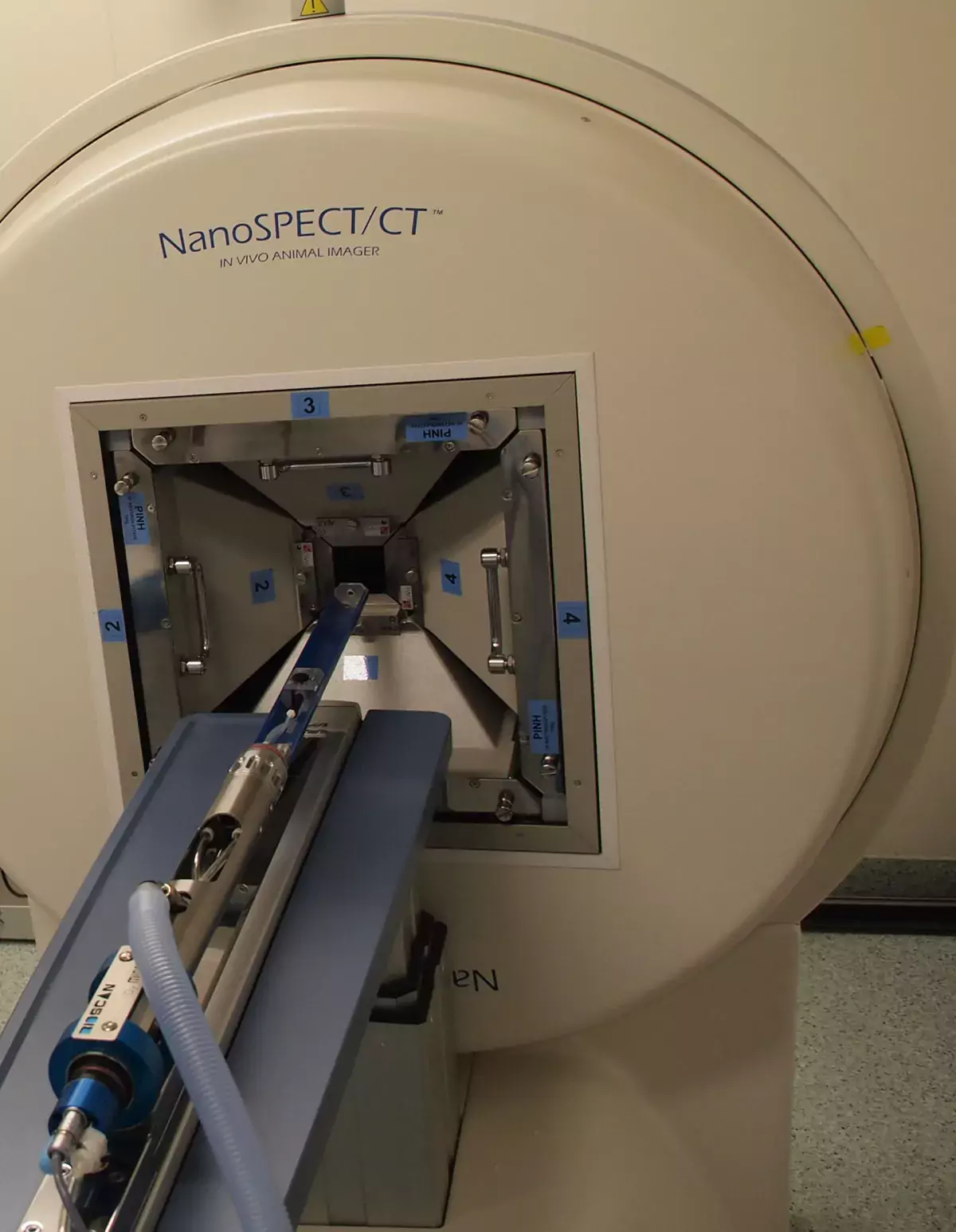
The Real-Time Imaging Unit (RTI) at Faculty of Pharmacy, University of Helsinki, provides multimodality imaging and image analysis for small animals. The RTI combines state-of-the-art instrumentation and radiotracer services to assist investigators with a wide range of imaging based experimental approaches. The RTI currently provides imaging modalities including single photon emission computed tomography (SPECT) and computed tomography (CT) (Fig. 1). In addition, dedicated housing is available for mice and rats undergoing longitudinal imaging studies. Ancillary facilities and resources of the RTI are devoted to radiopharmacy and image analysis.
Fig. 1. SPECT/CT scanner (NanoSPECT/CT, Bioscan, USA) at RTI located in BK3 Viikinkaari 1.
RTI is an imaging Core Unit of The Division of Pharmacology and Pharmacotherapy (DPP). Prof. Raimo Tuominen is the Head of the DPP and Docent J. Arturo García-Horsman is Group Leader of RTI.
RTI – Contacts:
J. Arturo García-Horsman, PhD | arturo.garcia@helsinki.fi | +358 50-4480761
Pharmacology and Pharmacotherapy
Viikinkaari 5E
FI-00014 UNIVERSITY OF HELSINKI
PRINCIPLES OF SPECT
SPECT is a three dimensional (3D) in vivo imaging method. Tracers labelled with radioactive isotopes are injected, ingested or inhaled into the small animal. The radioactive isotope decays, resulting in the emission of gamma rays. These gamma rays can be measured with the SPECT scanner to visualize functional information. Pre-clinical studies can be performed on animals, which are still alive after the measurement.
Functional in vivo imaging in animals is leading to accelerated studies of disease process and to development of drugs for diagnosing and treating a wide variety of conditions including several neurodegenerative diseases, and many types of cancer. Presently, with the advent of transgenesis and gene targeting the number of mutant mice strains available for biological research has greatly increased [1].
Recently, SPECT systems designed specifically for small animal imaging have become widely available. This nuclear imaging method has several characteristics which are well suited for drug research. SPECT has important advantages over other imaging modalities such as positron emission tomography (PET) [2].
SCANNERS
On the instrumentation side, SPECT systems lack anatomical resolution compared to ultrasound, CT and magnetic resonance imaging (MRI). However, multimodality imaging combines the functional information from SPECT with the anatomical detailing from CT. Hybrid SPECT/CT and PET/CT are in the clinical mainstream with many applications in fields including oncology and neurology. Nuclear medicine imaging is also often involved in the developmental process of therapeutic drugs. New chemical entities first need to comply with the proof-of-principle. For this purpose SPECT/CT imaging equipment has been designed for small animals, thus allowing a complete morphological and functional study of the targeting properties and overall pharmacokinetics in a pre-clinical setting [3].
Fig. 2. NanoSPECT/CT gantry
MicroCT imaging technology, which involves a series of X-ray images in 2- dimension that interact with the tissues of an animal. Tomographic 3D images are then reconstructed for visualization and analysis (Fig. 2).
SPECT is the most commonly used technique for clinical nuclear medicine imaging and it is also increasingly used in the field of animal research. This technique is especially qualified for accurate and non-invasive longitudinal studies of animal models of human physiology and disease because 3D visualization and accurate quantification of radioligands is possible. The technique is already commercially available from several companies. SPECT has financial benefits when compared to animal PET as well as offering a higher spatial resolution and the possibility to image different molecules in a single scan.
Table 1: Differences between imaging modalities, adapted from [4]
The use of SPECT in animal research has become increasingly practical. In addition to having a wide choice of relatively inexpensive radiopharmaceuticals, long-lived radioisotopes with half-lives from a few hours to a few days are also available for antibody-binding studies and serial studies. SPECT has additional capabilities as well, including dual-isotope imaging and the imaging of radioisotopes used for tumor therapy where the dose is delivered at the same time as image is produced. New modalities, such as SPECT/MRI, could offer the advantage of additional anatomic and functional information, generating the next level of multimodality molecular imaging (Table 1).
SPECT records gamma-rays directly after radionuclide emission, thereby gaining a theoretic advantage in spatial resolution over PET, for which resolution is currently limited by fundamental processes of positron emission and annihilation. With SPECT sub-half millimeter spatial resolution is achieved whereas in PET the spatial resolution is in the range of one millimeter.
Radiotracers
Radionuclides with relatively short half-lives are widely used in clinical nuclear medicine SPECT imaging because the rapid decay of radioactivity permits the administration of relatively large quantities to the patient. Same SPECT radionuclides are available for preclinical SPECT imaging applications. The most commonly used radionuclides are Tc-99m, In-111, and I-123. However there are wide spectra of other radionuclides that can be used in preclinical SPECT imaging [3].
For preclinical SPECT/CT studies, an ideal radionuclide should fulfill the following requirements:
The radionuclide should emit sufficient amount of photons (gamma rays) which can be detected outside the animal. Ideally the radioactive decay should be associated with photons with energy ranging between 100 and 250 keV, allowing adequate detection by pinhole SPECT.
The radionuclide should have a physical half-life in the order of hours, permitting longer follow up of labeled compounds in biodistribution studies and supplying with sufficient time for imaging.
The radionuclide should be able to tag to other chemical moieties of potential drug compounds.
The radionuclide should be readily available. Radionuclides that are produced with a radionuclide generator such as Tc-99m, are preferred over radionuclides produced with a cyclotron, which are usually more expensive.
Description of SPECT/CT Method for Small Animal Imaging
In Fig. 3 are shown the main processes of animal SPECT imaging [2].
Fig. 3. Illustration of the animal SPECT process.
SPECT can be combined with CT in an integrated small animal imaging system. SPECT/CT systems allow the radionuclide and CT data to be acquired and coregistered with minimal movement of the subject using acompletely automated image registration method. CT provides excellent gross anatomic localization. Once the images are recorded as accurately as possible within the constraints of the equipment, quantitative data that have physiologic relevance may be obtained.
Fig. 4. Multiplexing Multi-Pinhole (MMP™) SPECT
Multipinhole SPECT systems (Fig. 4) have greatly improved detection efficiency and permit submillimeter to millimeter spatial resolution to be achieved. With this model, the algorithm must reconstruct overlapping data to form the image. This model reduces scan time to a few minutes and less radiation to the animal is needed. The multidetector multipinhole SPECT, which can have up to 70 pinholes, is the most complex model. Other features of this model include detection efficiency nearing 1% (10,000 cps/MBq), scan times of 1 minute per frame or longer, “diagnostic” levels of radioactivity, and the possibility of multiple injections [5].
EXAMPLE OF A SPECT/CT EXPERIMENT
The following is an example of brain imaging experiment with rats using I- 123 tracer, b-CIT (I-123 half-life 13 h). This case result is shown in Fig. 5.
SPECT/CT calibration and QC procedures
Necessary calibrations have been performed before the experiment. Radiopharmacy (preparing the radiotracers)
I-123 b-CIT is a commercial tracer. In this case when the tracer have been delivered as an injection solution there were no other procedures than unpacking the radioactive material and dispensing the solution for i.v. administration.
Quality control of radiotracers
The vendor has been responsible for radiochemical and radionuclide purity of the tracer. There was no need for additional quality control procedure on site. Amount of radioactivity was measured by dose calibrator.
Administration of tracers (usually i.v. injection)
A dose of 40 MBq of I-123 b-CIT was administered i.v. in a volume of 1 ml in tail vein using a cannula. Radioactivity of syringe and cannula was measured after injection to find out the amount of injected radioactivity into animal.
Animal anesthesia
Animals were maintained under isoflurane gas anesthesia during operations (optional) and SPECT/CT imaging (Fig. 6).
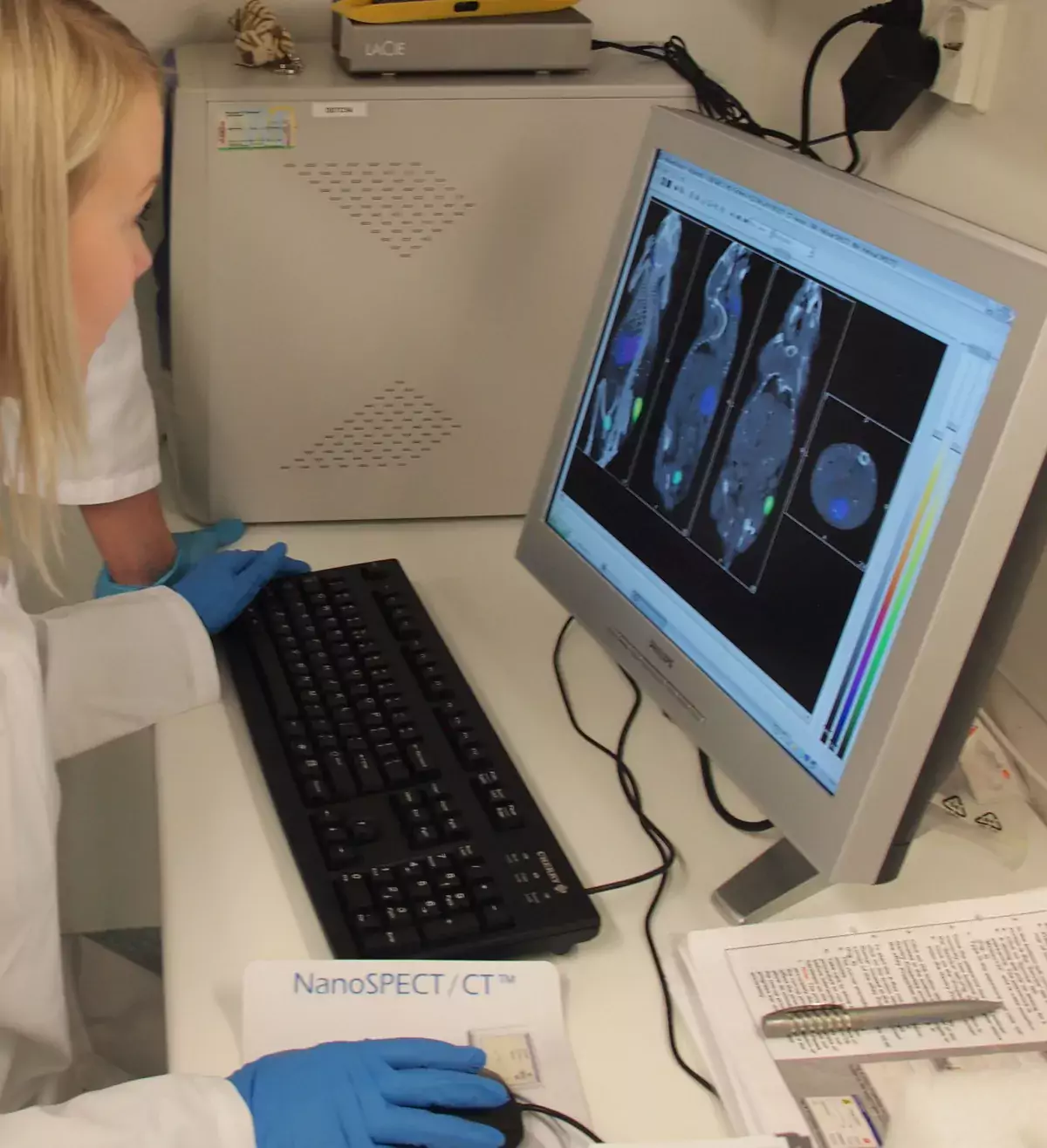
Fig. 5. SPECT/CT (NanoSPECT/CT, Bioscan, USA) imaging after i.v. injection of 38-40 MBq of [123I]b-CIT, a tracer which binds brain dopamine transporter. SPECT 264-317 min post-injection. SPECT colour scale (white-yellow-red-blue).
Fig. 6. Animal set-up before scanning
SPECT/CT scan
SPECT images were obtained in 24 projections using four-headed detectors with multipinhole collimators over 30 min. CT images were obtained over 5 min.
SPECT/CT data analysis
SPECT images were reconstructed using propriety iterative reconstruction and fused with CT images.
WORKING SPACES AT RTI
RTI Unit is located in the Laboratory Animal Centre of Biocenter 3 at Viikinkaari 1. Core facilities include type B radioisotope laboratory and biosafety class 2 facility. In Figs 7-10 some of the equipments of the Unit are shown.
Fig. 7. SPECT/CT scanner room showing NanoSPECT/CT (Bioscan Inc.), Laminar- flow cabinet and dose calibrator.
Fig. 8. Gas anesthesia unit for use of isoflurane with oxygen carrier (left) and a rat in the induction chamber before scanning (right). Anesthesia unit is also connected to scanner animal bed and bench mask.
Fig. 9. Radioactive material storage (left) and a working space (right) to handle radioactive material behind lead glass.
Fig. 10. Scantainer with lead glass to keep animals, and radioactive contamination monitor.
EXAMPLES OF SPECT APPLICATIONS
There are several possible applications and examples where SPECT is exploited in literature. State-of-the-art SPECT/CT devices give a better possibility to apply SPECT method in a new way. Different radionuclides may be used for labelling small size molecules (usually >400 g/mol), biomolecules (peptides, proteins etc.), and particles (nanoparticles, cells etc.) [2]. In Fig. 12 are shown some of the applications [5].
Fig. 11. (a) Mouse bone metabolism study; (b) Detection of NIS reporter gene expression in one tumor with negative control in another ; (c) Detection of pancreas tumor metastasis by imaging of elevated gastrin levels in a mouse by using Tc-99m;
(d) Study of Parkinson disease in the mouse brain by Dopamine-D2 receptor imaging with I-123-IBZM; (e) Illustration of the dramatic improvement in image detail of mouse kidneys by going from “micro-” to “nano-” precision imaging [5].
REFERENCES :
Kairemo K, Bergström K. The Role of Radiopharmaceuticals in Drug Discovery; Guest Editors: K. Kairemo and K. Bergström. Curr Radiopharmaceuticals 2008; 1(1):1.
Franc BL, Acton PD, Mari C, and Hasegawa BH. Small-Animal SPECT and SPECT/CT: Important Tools for Preclinical Investigation. J Nucl Med 2008; 49:1651–1663.
Pauwels EKJ, Bergström K, Mariani G, Kairemo K. Microdosing, Imaging Biomarkers and SPECT: A Multi-Sided Tripod to Accelerate Drug Development. Curr Pharmaceutical Design 2009; 15:928-934.
Kairemo K, Erba P, Bergström K, Pauwels EKJ. Nanoparticles in Cancer. Curr Radiopharmaceuticals 2008; 1(1):30-36.
Van Cauter SC. In vivo Animal Imaging goes Nano: Now You Can Image a Mouse like a Man . Bioscan Inc., Washington, D.C.